诺和泰® Ozempic®
展商:Novo Nordisk
原产国/地区:丹麦
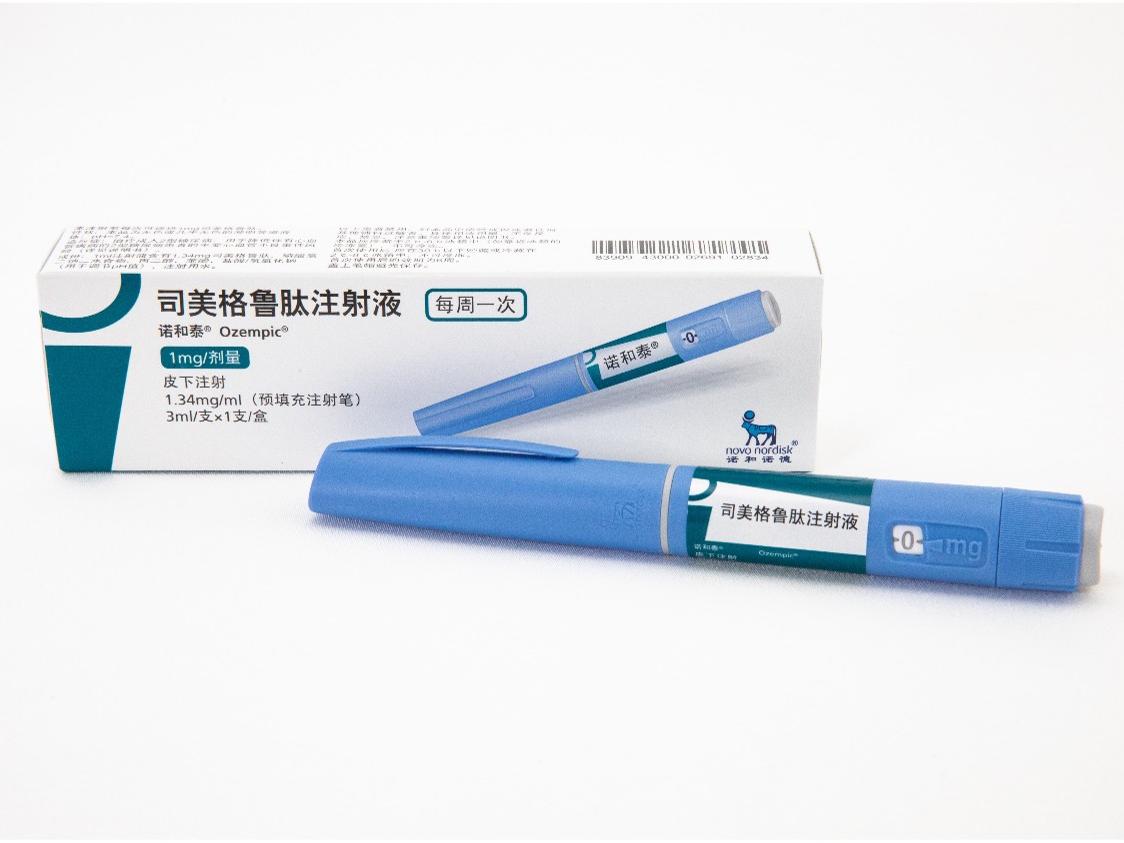
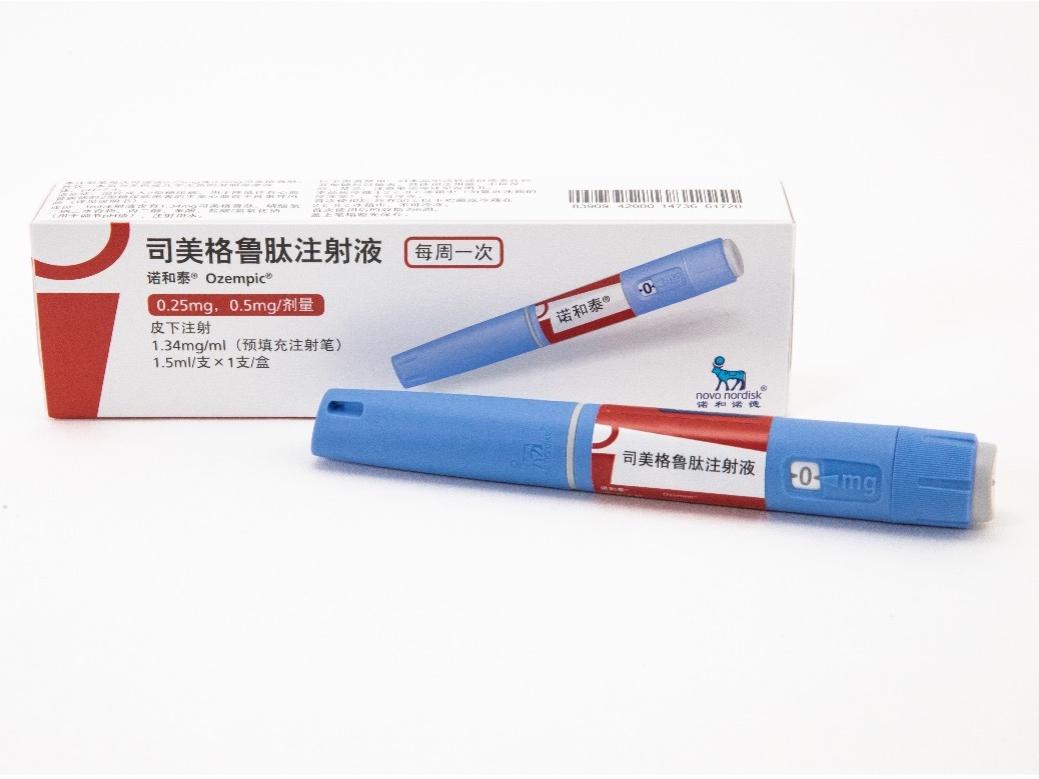
活性成分:司美格鲁肽,1ml注射液中含有1.34mg司美格鲁肽。每支预填充注射笔含有司美格鲁肽2mg,置于1.5ml溶液中。 本品适用于成人2型糖尿病患者的血糖控制:在饮食控制和运动的基础上,接受二甲双胍和/或磺脲类药物治疗血糖仍控制不佳的成人2型糖尿病患者。 适用于降低伴有心血管疾病的2型糖尿病成人患者的主要心血管不良事件(心血管死亡、非致死性心肌梗死或非致死性卒中)风险。
展品详情
产品:诺和泰®(司美格鲁肽注射液)
介绍:
诺和泰®(司美格鲁肽注射液)是通过基因重组技术,利用酿酒酵母细胞生产的人胰高血糖素样肽-1(GLP-1)类似物,具有94%的序列同源性。
司美格鲁肽已经在中国、美国、欧盟、加拿大、日本等多个国家及地区获批上市。2021年4月27日,国家药品监督管理局(NMPA)批准了诺和泰®(司美格鲁肽注射液)的上市申请,用于成人2型糖尿病患者的血糖控制,同时还可用于降低伴有心血管疾病的2型糖尿病成人患者主要心血管不良事件风险。
诺和泰®具有下列显著特点:
强效:诺和泰®在中国人群中糖化血红蛋白达标率高达86.1%,表现出非常好的降糖疗效。
多效:诺和泰®是中国首个且目前唯一具有心血管适应症的GLP-1周制剂,为2型糖尿病患者带来心血管保护和减重、降压、调脂等心血管代谢综合获益。
长效:诺和泰®通过突破性肽链结构优化,将半衰期延长至7天,实现了一周一次用药,血药浓度非常平稳。
诺和泰®于2021年纳入国家医保目录,并于2022年1月执行。
Product: Ozempic® (Semaglutide Injection)
Introduction:
Ozempic® (Semaglutide Injection) is a human glucagon-like peptide-1 (GLP-1) analogue produced in Saccharomyces cerevisiae cells by recombinant DNA technology with 94% sequence homology to human GLP-1.
Semaglutide has been approved and launched in many countries and regions around the world, including China, the European Union, the United States, Canada and Japan etc. Ozempic® got NMPA’s approval on 27th April 2021 with generic name of Semaglutide Injection. Ozempic® could offer optimized antidiabetic treatment choice for T2DM patients in China, and also could be used to reduce CV major adverse cardiovascular events(MACE)risk for T2DM patients.
Ozempic® has the following distinctive features:
Superior efficacy: HbA1c target(<7%) achieving rate of Ozempic® in Chinese T2DM patient is as high as 86.1%, showing good efficacy.
Cardio-metabolic benefits: Ozempic® is the only once weekly GLP-1RA with CV indication approved in China. Ozempic® brings CV protection and more other cardio-metabolic benefits such as weight loss, blood pressure and lipid profile improvement for Chinese T2DM patients.
Long acting: Through optimization of peptide chain structure, Ozempic® successfully extend half-life to 7 days, achieving stable plasma concentration with once weekly injection.
Ozempic® has been listed in National Reimbursement Drug List and effective on January 1st, 2022.