News Center

CIIE Stories | CIIE accelerates medical innovation, market access Release date: 2024-03-19 Source:China International Import Expo Bureau
Since its inaugural edition in 2018, the China International Import Expo has emerged as a vital platform for showcasing innovative medical solutions, including treatments for rare diseases, and driving global medical enterprises to innovate and invest in China.
Leveraging the CIIE, numerous innovative drugs and medical devices have entered the Chinese market, providing Chinese doctors and patients with a plethora of new treatment options.
Siemens Healthineers' Naeotom Alpha, the world's first photon-counting CT scanner, has become commercially available in China just two years after its debut at the fourth CIIE.
Jerry Wang, head of China at Siemens Healthineers, said: "The CIIE has garnered attention from multiple parties, including medical products and healthcare security authorities, facilitating the expedited introduction of innovative medical devices."
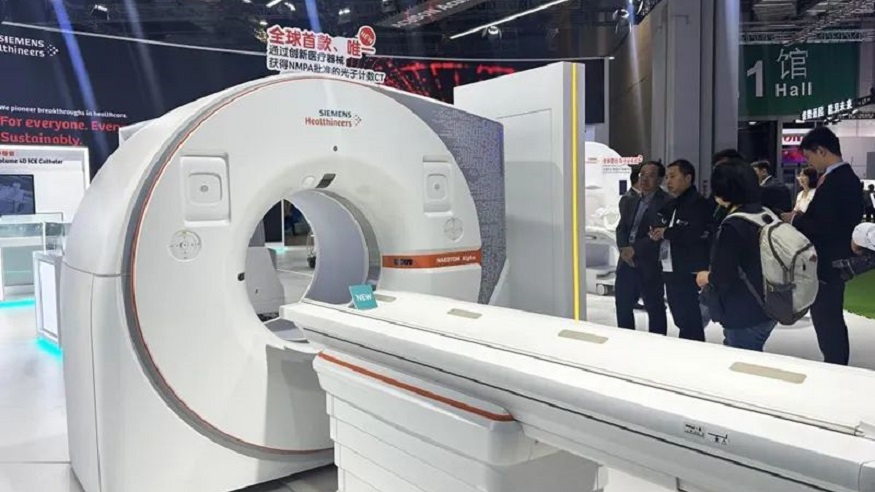
Siemens Healthineers' Naeotom Alpha is showcased at the sixth CIIE. [Photo/Xinhua]
Gilead Sciences' AmBisome, a drug for treating invasive fungal infections, was showcased at the fifth CIIE. It similarly received approval from China's National Medical Products Administration to enter the market just three months after the expo.
The CIIE has also played a significant role in accelerating the approval process for new indications of existing drugs.
Japanese pharmaceutical company Takeda's Revestive®, which is used for treating short bowel syndrome, was also featured at the fifth CIIE. Within six months after the expo, it entered trial use in medical institutions in the Boao Lecheng International Medical Tourism Pilot Zone in Hainan and received official approval from the NMPA just 15 months after the expo.
In addition to facilitating the transition of exhibits into commercial products, the CIIE has also prompted exhibitors to boost investment in China and expedite their localization strategies.
For example, Boston Scientific's Polaris, a PCI (percutaneous coronary intervention) guiding device, caught the attention of Shanghai's drug regulatory authority at its CIIE debut in 2019. In July 2022, the company obtained a license to produce the device in China and the first Polaris then rolled off the line in a Shanghai factory in September of the same year.
Through the CIIE, certain exhibits have also earned inclusion in China's national medical insurance catalog, which benefits the Chinese people and contributes to the Healthy China 2030 Initiative.
At the sixth CIIE, biopharmaceutical company GSK showcased its Benlysta for the fifth consecutive year. During the five years, Benlysta has been included in China’s medical insurance coverage list to treat systemic lupus erythematosus in adults and children.
Vocinti, another CIIE exhibit from Takeda, used in treatment for reflux esophagitis, was also included in China's medical insurance coverage list in 2020.
"We will accelerate the introduction of more flagship products in China and actively participate in building China's medical innovation ecosystem,” said Sean Shan, senior vice-president of Takeda and president of Takeda China, adding that the company will also empower the high-quality development in Chinese healthcare and contribute to the realization of Healthy China 2030 Initiative.

Benlysta is showcased at the second CIIE. [Photo/The Paper]
By Zhao Guangmei